ASPERGILLUS GALACTOMANNAN Ag VIRCLIA®
Invasive Aspergillosis: The Urgency of Rapid and Accurate Diagnosis
Invasive Aspergillosis is a severe infection caused by the Aspergillus fungus, which primarily affects individuals with weakened immune systems, such as those undergoing chemotherapy, organ transplants, or suffering from advanced HIV/AIDS. Unlike mild forms of aspergillosis, the invasive type can spread quickly from the lungs to other parts of the body, including the brain, kidneys, and heart, making it life-threatening.
The importance of rapid and accurate diagnosis cannot be overstated, as delays in identifying and treating invasive aspergillosis can result in high mortality rates. Early detection allows for timely antifungal treatment, improving outcomes and significantly reducing the risk of complications. Given its rapid progression, accurate diagnostic tools are crucial for effectively managing the infection in vulnerable patients.

Save time, save lives
> No sample accumulation
> The true monotest
1 sample= 1 reportable result. No waste of reagents
Individual quality control per monotest

> Excellence performance against the gold standard in the market
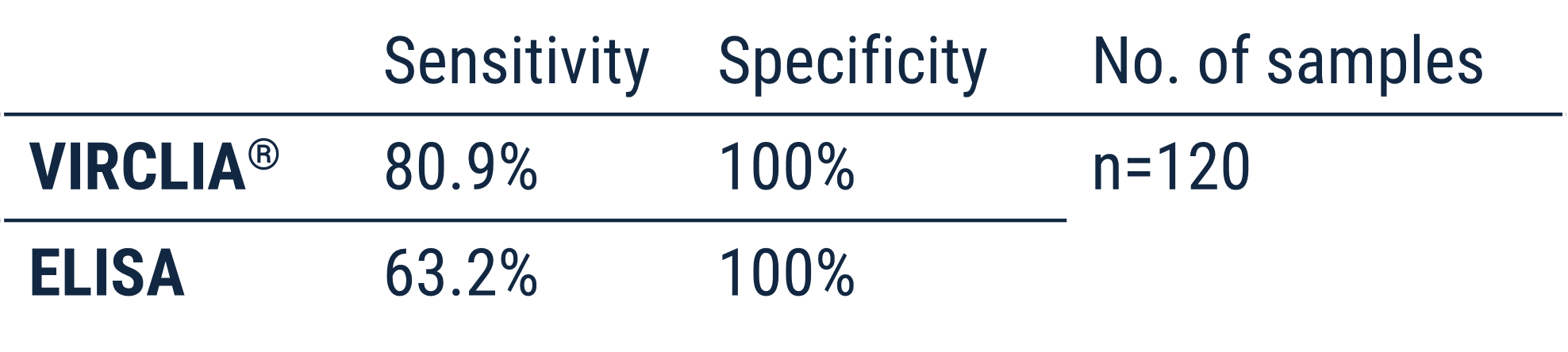
Internal evaluations show great diagnostic perforamance against a commercial ELISA in a high number of samples.
In a recent publication versus a commercial ELISA as a reference, VIRCLIA showed excellent results:

Within this study, 120 patients had criteria for proven or probable IA according EORTC/MSGERC criteria:
> Fully automated protocol with VirClia® Lotus
Combine with more than 90 parameters for infectious diseases.
Join the Revolution in Infectious Serology
Publications and Media

Retrospective Multicenter Evaluation of the VirClia Galactomannan Antigen Assay for the Diagnosis of Pulmonary Aspergillosis with Bronchoalveolar Lavage Fluid Samples from Patients with Hematological Disease
Buil et al., Journal of Clinical Microbiology, 2023
The VirClia Galactomannan (GM) assay demonstrated diagnostic accuracy comparable to the Platelia GM assay, with sensitivity and specificity closely aligned. It offers faster, on-demand testing of bronchoalveolar lavage (BAL) fluid samples without batch processing. The assay is a reliable tool for diagnosing invasive pulmonary aspergillosis (IPA) in patients with hematological diseases.

Comparative performance of the Platelia Aspergillus Antigen and Aspergillus Galactomannan antigen Virclia Monotest immunoassays in serum and lower respiratory tract specimens: a “real-life” experience
Albert et al., Clinical Microbiology, 2024
This study compared the Platelia and VirClia Aspergillus Galactomannan assays using 535 consecutive fresh samples. The VirClia assay showed higher sensitivity, detecting more positives, especially in respiratory samples like bronchoalveolar lavage and tracheal aspirates.

The Aspergillus galactomannan Ag VIRCLIA® Monotest and the Sona Aspergillus galactomannan lateral flow assay show comparable performance for the diagnosis of invasive aspergillosis.
The VirClia Galactomannan Monotest and Sona Galactomannan lateral flow assay demonstrated similar diagnostic accuracy for invasive aspergillosis, with comparable sensitivity for bronchoalveolar lavage (BAL) samples. The VirClia Monotest provided better reproducibility and faster processing times. It is a practical choice for facilities requiring individual sample testing.
Vircell in Fungal Congresses
Advances Against Aspergillosis and Mucormycosis
Vircell is proud to have participated for several years in the Advances Against Aspergillosis & Mucormycosis Conference. This prestigious event brings together leading fungal experts from around the globe.
Trends in Medical Mycology | 19-22 September 2025
The 12th Trends in Medical Mycology (TIMM-12) will be held in Bilbao, Spain. It will gather over 1,000 experts to discuss fungal biology, diagnostics, and antifungal resistance. TIMM offers networking and insights into both research and clinical application.
Balkan Fungus 2024 | 10-12 October
Discover the advantage of VirClia ® Lotus for fungal infections
Other Events
Check our upcoming events
Technical Specifications
Sandwich Chemiluminescent Immunoassay (CLIA) for the detection of Aspergillus galactomannan antigen in serum, plasma and human bronchoalveolar lavage (BAL) samples.
- Same day results, no batching, no sample cumulation.
- On-demand testing: One monotest = one reportable result. Nothing else is required.
- Simple and fully automated protocol with results in about 1h 15minutes, sample pretreatment needed.
- Objective method with reported results in index values.
- Compatible test with the broadest panel of infectious diseases in CLIA monotest format (>90 parametres).




