Coxiella burnetii, a complex diagnosis
Coxiella burnetii and Q fever: a silent threat
Q fever is a zoonotic disease caused by Coxiella burnetii (C.burnetii), an intracellular bacterium with a global distribution. It infects a wide range of animals, including livestock, wildlife, and tick species that act as vectors. Human infection usually occurs through the inhalation of infectious aerosols or contaminated dust from infected animal fluids such as urine, feces, milk, or birth products.
While Q fever is often asymptomatic or mild, some cases progress to severe forms such as atypical pneumonia, hepatitis, or, in chronic stages, endocarditis.
Two antigenic phases: Key to Diagnosis
Coxiella burnetii presents two distinct antigenic forms:
- Phase I: The highly virulent form with a complete lipopolysaccharide (LPS) structure. It is associated with chronic infections such as endocarditis.
- Phase II: A less virulent form with a modified LPS, predominant in acute infections.
Serological differentiation of these phases is essential for determining disease progression.
Challenges in Q fever diagnosis
Since isolating Coxiella burnetii in culture is difficult and highly infectious, serological methods are the primary diagnostic approach.
Key diagnostic challenges:
- Early-stage infections often yield false-negative results.
- Detecting phase II antibodies alone does not distinguish between acute and chronic infections.
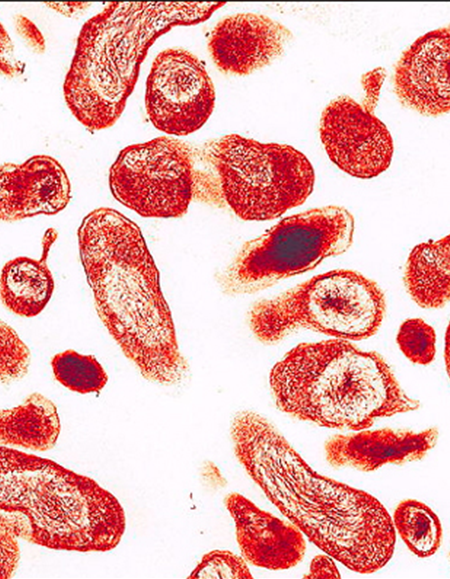
- The gold standard, indirect immunofluorescence assay (IFA), is sensitive but requires trained personnel.
Vircell’s Solutions for Coxiella burnetii

IFA – Gold Standard for Comprehensive Diagnosis
COXIELLA BURNETII IFA
- Complete panel: IgG, IgM, IgG/IgM/IgA (phase I and phase II)
- All the necessary reagents included in the kit
- The PCOBU I+II reference allows to discriminate between acute and chronic Q fever. Phase I and phase II antigens are separated in different wells to facilitate the reading of results.
- Many traditional suppliers have stopped offering this parameter (IFA), and Vircell is now one of the few available alternatives.
ELISA – Automated Rutinary Testing
Indirect immunoenzyme assay to test antibodies against Coxiella burnetii phase II in human serum/plasma.
- Serum dilution and sorbent treatment, when necessary, directly in the well.
- Suitable for automated ELISA systems.

VirClia® – The Only CLIA Monotest for C. burnetii
Indirect chemiluminescent immunoassay to test antibodies against Coxiella burnetii phase II in human serum/plasma.
- Monotest format with ready-to-use reagents.
- Simple and automated protocol with fast results.
- Custom setup of samples and reagents.
- Each monodose includes a calibrator and a negative control that enables the validation and interpretation of results for each individual sample
- Allows pipetting from primary sample tube, no further manipulation is needed
Amplirun® – Complete DNA control for Coxiella burnetii detection
Purified DNA of Coxiella burnetii designed to support molecular workflows and validate nucleic acid amplification techniques (NAATs).
- Contains purified, complete microbial genome – any sequence can be amplified
- Ideal for assay development, validation, and quality control
- Compatible with any molecular testing platform
- Non-infectious and safe to handle
- Lyophilized format for extended shelf life and easy transport
Technical Comparison Between IFA and VirClia®
IFA (Immunofluorescence)
Multi-well slide
Manual technique requiring multiple incubation and washing steps.
Phase I and II
Subjective reading under a fluorescence microscope, requiring experienced personnel.
Gold standard but dependent on technician expertise.
VirClia® (CLIA Monotest)
Multi-Single-dose automated testplate
Fully automated with a simple, standardized protocol.
Phase II
Automated and objetive, reducing operator variability
High sensitivity with automated and reproducible results.

