NEW PRODUCT VIRAPID® TULAREMIA
Vircell launchs new reference for the diagnosis of tularemia
Vircell is happy to announce the launch of a new product within its immunochromatographic tests line: VIRapid® TULAREMIA (ref. VR006) for the qualitative detection of total antibodies against Francisella tularensis in both serum and plasma samples.
Tularemia is a highly infectious disease caused by the bacterium Francisella tularensis. Human infections are often result from contact with infected wildlife, ingestion of or contact with contaminated water, or bites from ticks and other arthropods that have fed on infected wildlife.
Disease is expressed in different clinical forms, and varies in severity depending on the virulence of the organism, dose, and site of inoculums. In humans, tularemia, especially the milder forms, is also undoubtedly under recognized and underreported. Nevertheless, reported human cases provide sufficient information for evaluating patterns and trends for this disease.
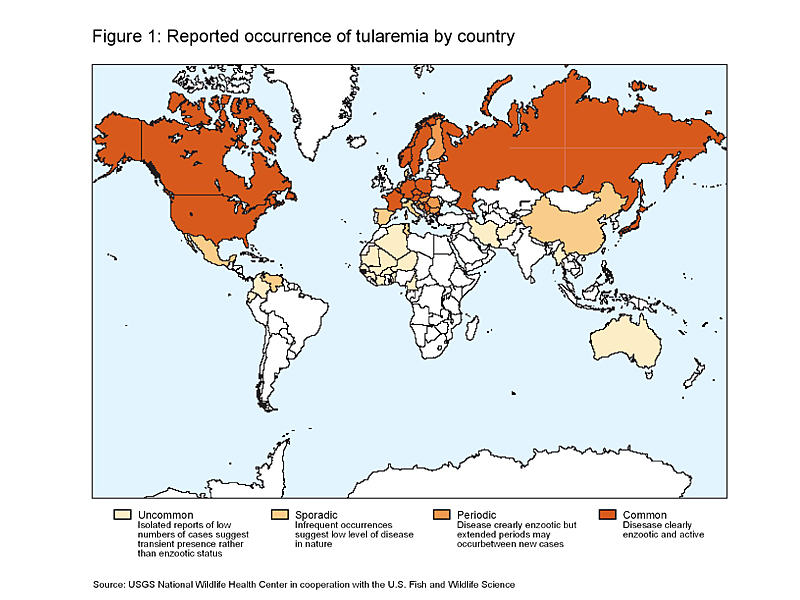
Tularemia occurs throughout much of North America and Eurasia (Figure 1) but has a patchy distribution in the Northern Hemisphere. Although the disease is widely endemic in Eurasia, it is typically a disease of Northern and Central Europe; Scandinavia and countries of the former Soviet Union report the most human cases. In Asia, tularemia is most prevalent in Japan.
In humans, tularemia is often diagnosed by serology, as the isolation of the organism can take from 2 days to more than 2 weeks and must be done at biosafety level 3, which is not available in all diagnostic laboratories. PCR can also identify F. tularensis in clinical samples, however, specialized instrumentation is required and the prices are usually high.
VIRapid® TULAREMIA is the first immunochromatographic test in the market for Francisella tularensis detection and offers important benefits for both, big and small laboratories, in prevalent areas against the techniques currently used:
- Excellent performance (99.13% sensitivity and 98.58% specificity)Based on LPS (conjugate and test line). Total antibody detection (IgG+IgM+IgA)
- Rapid detection - visual reading in just 15 minutes. No hazardous diagnosis
- User-friendly protocol with little manipulation- No special instrumentation nor qualified personnel is required to perform the test
- All necessary reagents included in the kit
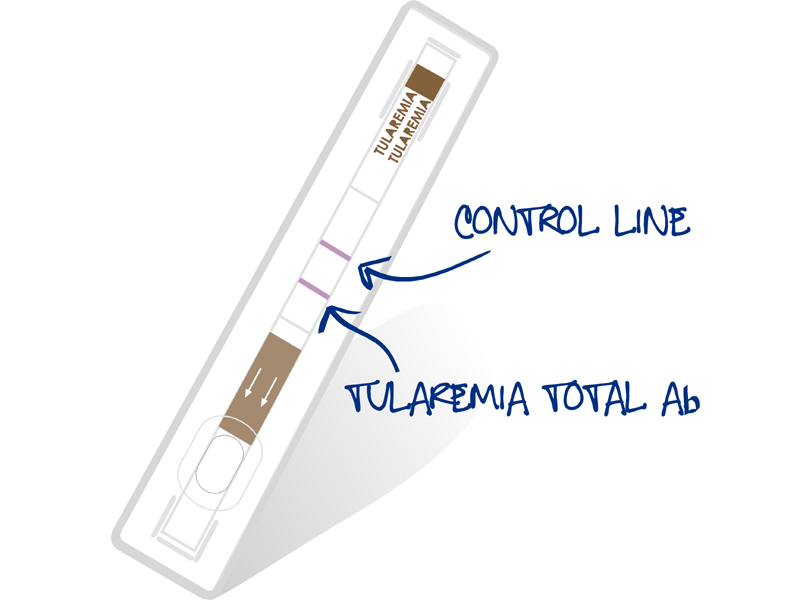
- Product control line on each device
- Room temperature storage
- CE marked (IVD)
For further information, do not hesitate to contact us.